IRB Administrator Assignments
Generally, each new RAMP IRB submission is upon triage assigned to a specific IRB Administrator (coordinator) or other staff to complete Pre-Review and as applicable prepare and manage the submitted study for IRB review. Subsequent submissions for a cleared or IRB-approved study are generally routed by the RAMP IRB system to the coordinator currently assigned to the study. Note that once submitted and at any time during the life cycle of a study, the assigned IRB Coordinator is subject to change.
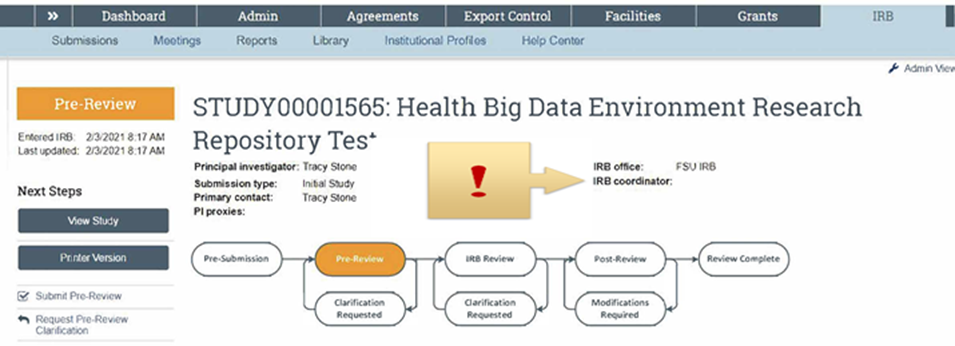
To check the IRB Coordinator currently assigned to any of your RAMP IRB studies, log into RAMP IRB and retrieve your study in question. Above the workflow diagram, the name of your IRB Coordinator will if assigned be shown (see figure below). Always check this assignment so that you know who to contact if you have questions.
For questions about a RAMP IRB submission for which an IRB Coordinator is or may not be currently assigned, use the Add Comment feature under Next Steps (see figure below) to submit a question (do not use the Add Comment feature to submit study materials; use the Edit Study feature instead). Alternatively or for a planned study that you have not yet submitted, use the general email at humansubjects@fsu.edu to ask your question, and staff will follow up with you.