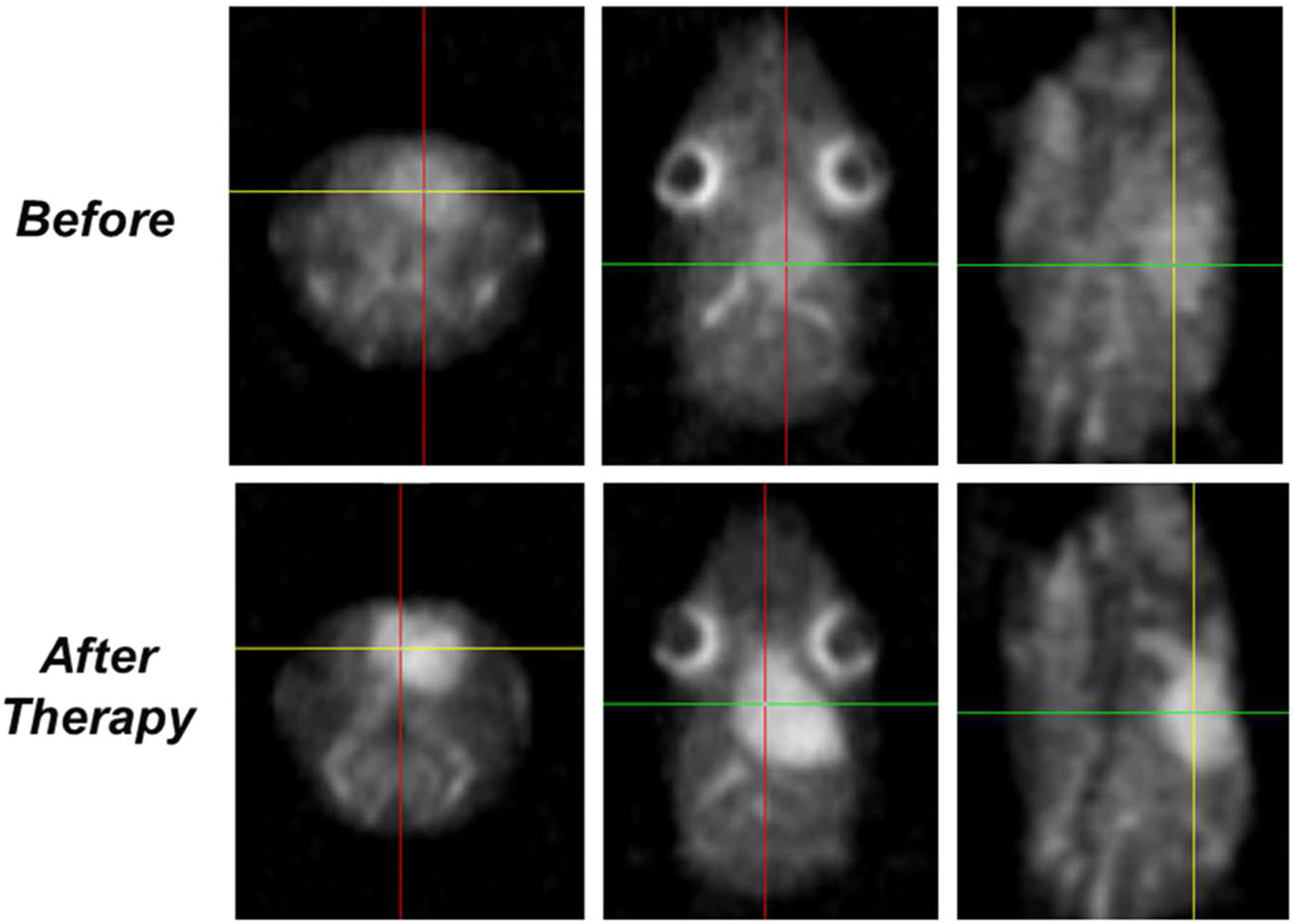
Tumor Drug Resistance Measured by Sodium Diffusion
This invention is a non-invasive, comprehensive and individualized evaluation of tumor resistance using sodium and/or diffusion magnetic resonance imaging (MRI). The method includes conducting a sodium and/or diffusion MRI on a tumor of a subject and on a normal region of the subject- for example, the normal region in brain being contralateral to the tumor. When the images of the MRI procedures have been obtained, the indicias (i.e., sodium and/or diffusion) are measured and analyzed. These indicias are compared between the tumor region and normal region. A low level of the indicia in the tumor region, relative to the level of indicia in the normal region, indicates a higher/increased tumor resistance to a drug.
Currently, a biopsy and Positron emission tomography (PET) are the conventional technologies used to deliver information on tumor resistance prior to therapy. The evaluation can be performed prior to therapy and can help select a strategy of treatment but also help in evaluating the efficacy of an agent for the treatment of cancer in a subject. The invention can be used in the brain glioma model but is contemplated for use in different types of tumors in most parts of the human body in addition the agent may be carmustine, though other tumor types and agents are contemplated by the invention. The level of tumor resistance can be determined reproducibly in a relatively short amount of time, for example less than thirty minutes, and the results can be used immediately to create individualized therapy.
The invention allows clinicians to avoid ineffective therapies, which may be more harmful than useful or come up with the other more appropriate alternatives. It can facilitate a separation of the effects due to metabolic changes in the tumor at the beginning of therapy from the effects introduced by drug intervention.