Traceless directing groups in radical cascades: from oligoalkynes to fused helicenes without tethered initators
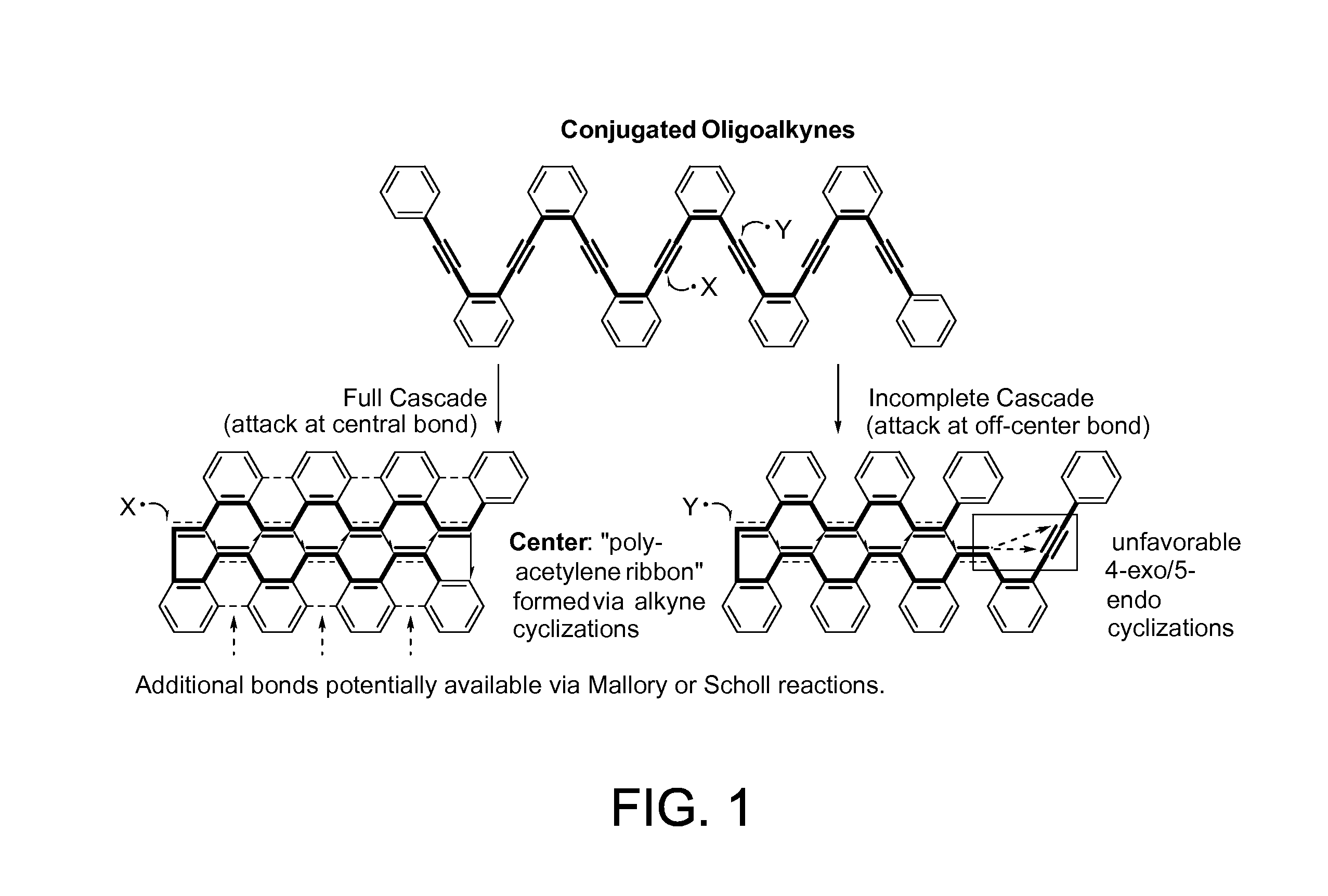
Dr. Alabugin and his team have developed a traceless directing group in a radical cascade. The chemo- and regioselectivity of the initial attack in skipped oligoalkynes is controlled by a propargyl alkoxy moiety. Radical translocations lead to the boomerang return of radical center to the site of initial attack where it assists to the elimination of the directing functionality via β-scission in the last step of the cascade. In some aspects, the reaction of the present invention is catalyzed by a stannane moiety, which allows further via facile reactions with electrophiles as well as Stille and Suzuki cross-coupling reactions. This selective radical transformation opens a new approach for the controlled transformation of skipped oligoalkynes into polycyclic ribbons of tunable dimensions.