Preparation of Expanded Polyaromatics
Current methods utilized to synthesize crowded polyaromatic architecture often use strategies that demand stringent design to achieve control over the size and substitution of the product. The proposed technique addresses this challenge by using a robust and flexible cyclization method in which a functional handle is installed during the reaction sequence to offer means for further extensions and functionalization.
The present invention is an efficient process to prepare synthetically challenging large distorted aromatics. The new approach developed at Florida State University efficiently transforms enynes into polyaromatic structures of precise dimensions and tunable electronic properties, solving the problem of selectivity in cascades aimed at the preparation of polyaromatic structures from conjugated enynes.
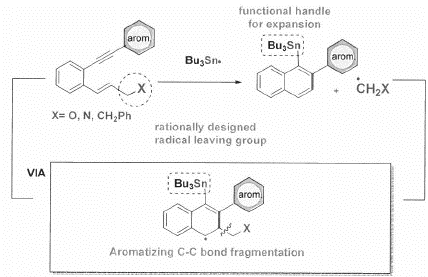
The overall process incorporates an unprecedented sequence in which chemo-and regioselective interaction of the triple bond with Bu3Sn radicals originates from a conceptually novel source and propagates in such a way that renders alkenes synthetic equivalents of alkyns. By coupling the cyclization/rearrangement cascade with an aromatizing C-C bond fragmentation, the net result is a convenient transformation of readily available enyne reactants to a-Sn substituted naphthalenes that can serve as a lauching platform for the preparation of extended distorted polyaromatics.
The key challenge that had remained in the design of radical cascades was achieving control over chemoselectivity of initial radical attack and the subsequent cyclization mode. We resolved these problems by using the first radical enyne cascade in which chemo- and regioselective interaction of the triple bond with Bu3Sn radicals originates from a novel 1,2 metallotropic shift.
The use of alkenes assists in the elimination of a radical leaving group via scission at the end of the cascade, aromatizing the final product without the need for external oxidants. This selective radical transformation opens a new approach for the controlled transformation of enynes into polycyclic distorted aromatics of tunable dimensions.
Advantages:
- The feasibility with which the scission of strong C-C bonds is accomplished under mild conditions.
- Provides a convenient and efficient method to synthesize large distorted aromatics and polycyclic ribbons of tunable dimensions.
- Installation of Bu3Sn at a specific position and conversion of readily available enynes into highly valuable a-Sn naphthalene derivatives in high yields in a single cascade step